Abstract
Background: Multiple myeloma(MM) remains incurable as most patients endure multiple progressions. The complexity of tumor-associated environment(TME) in the progression of MM was not fully clarified. We investigated the atlas of TME during the process of disease progression and explored potential novel therapeutic target.
Methods: We performed in-depth single-cell RNA sequencing(scRNAseq) analysis on the bone marrow(BM) cells from 14 MM patients at different disease stage and validate the findings from scRNAseq analysis by bulk RNAseq, flow cytometry analysis and in vitro & in vivo functional experiments.
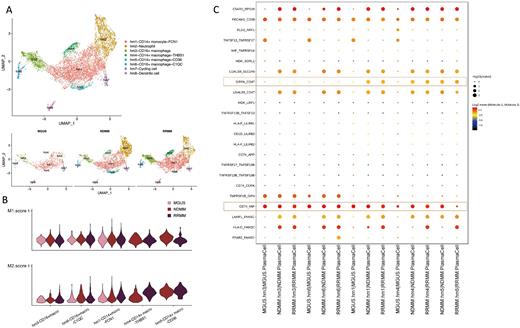
Results: We delineated a compromised TME during progression, characterized by abnormal enrichment of NK cells and exhausted-CD8+T cells, and reprogramming of macrophages(MΦs). A significant enrichment in NK cells and a decrease in CD34+ hematopoietic cells were observed, and the enrichment in NK cells was associated with the progression of disease stage. We found CD8+T cells expressing LAG3 and TIGIT were enriched in advanced disease stage. We further dissected the gene expression signatures of myeloid lineage cells and identified five monocyte/macrophage subsets, which were further divided into CD14+subsets (hm1, hm4, hm5) and CD16+subsets (hm3, hm6) (FigureA). In previous studies reported by other researchers, macrophages presenting M2 phenotype were highly enriched in the BM microenvironment and contributed to the progression of MM. We calculated M1/M2 polarization score for monocyte/macrophage clusters (Figure B). The gene expression signatures of the five macrophage clusters did not fit with either the canonical M1 or M2 classifications, demonstrating a reprogrammed phenotype rather than the M1/M2 paradigm. However, the two CD14+subsets (hm4 and hm5) only present in myeloma stage demonstrated high M2 score than other macrophage clusters (hm1, hm3 and hm6), implying the potential role of M2 polarization in myeloma progression. TAMs reprogramming was correlated with MM progression, mainly involving the CD14+subsets identified in late-stage-disease, featured by a prone-M2 phenotype and phagocytic dysfunction. Transcription factors associated with macrophage M2 polarization and immunosuppression were preferentially expressed in different TAMs clusters. We validate the reprogrammed phenotype of macrophages found in scRNAseq data by flow cytometry analysis and bulk RNAseq in patients samples and in vitro cell experiments. To interrogate global cell-cell interactions in myeloma during disease progression, we performed computational modeling using 'CellPhoneDB' to identify interactions between different monocyte/macrophage subclusters and plasma cells (PCs) in the BM microenvironment from different stage disease(Figure C). We identified two significantly enriched ligand-receptor pairs including the SIRPA-CD47 'don't eat me' pathway suppressing phagocytosis and the CD74-MIF(macrophage inhibitory factor) reshaping the MΦs phenotype. As CD47 and MIF molecules are linked with disease progression and adverse outcomes, we proposed the hypothesis that dual immunosuppression of TAMs by CD47 and MIF promotes disease progression in MM. Finally, we designed a dual-MΦs-targeted therapeutic strategy by combining anti-CD47 mAbs and MIF inhibitor to activate phagocytosis and repolarize MΦ to a functional phenotype, and proved the potent anti-tumor effect in vitro and in vivo.
Conclusion: We identified compositional alterations in MM-TME during the disease progression and unraveled significant TAMs phenotype reprogramming and phagocytic dysfunction. We discovered critical dual cellular cross-talks (CD47 and MIF) between MΦs and PCs, and explored their function and clinical significance. Finally, we designed a dual MΦs-targeted therapeutic strategy and proved its anti-tumor effect and the potential clinical benefits.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.